Advancing toward more tolerable solutions
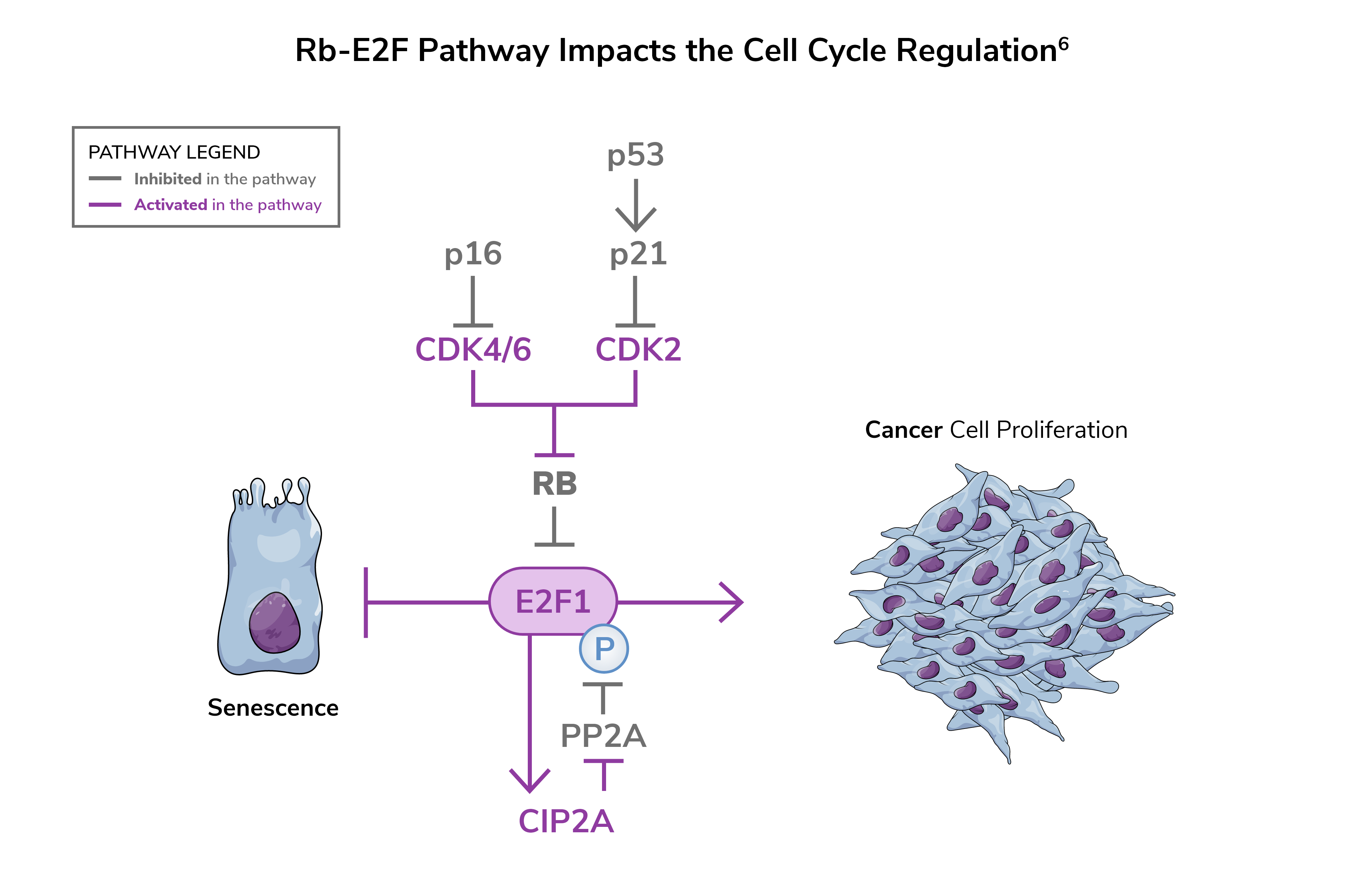
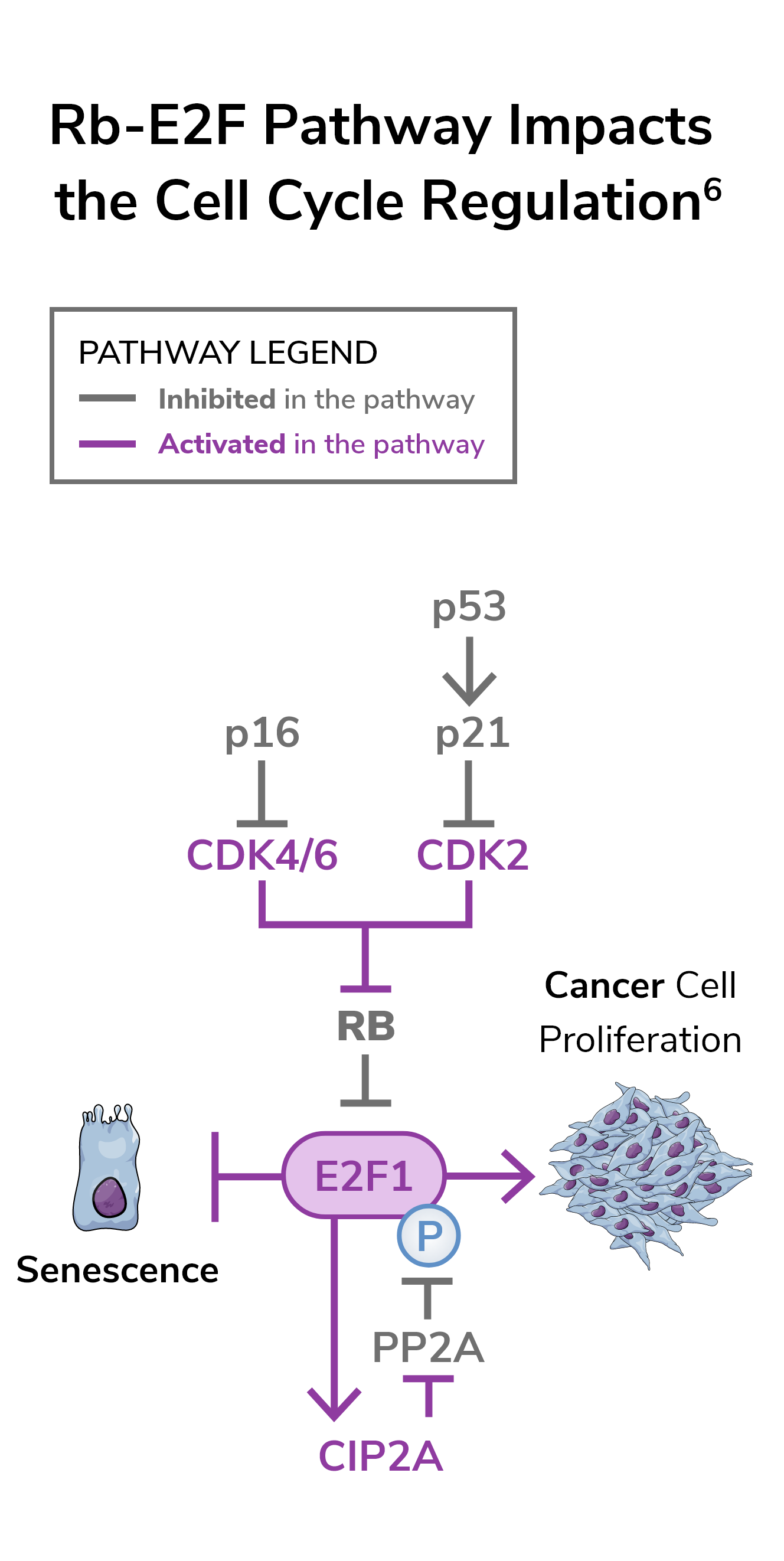
The Rb-E2F pathway is a critical regulator in the underlying biology of bladder cancer1
When functioning normally, Rb-E2F acts as a brake to prevent cell proliferation.2 Loss of functionality leads to loss of cell cycle control.3
The Rb-E2F pathway is altered in many cancers, including bladder cancer, which has been associated with progression, high risk of recurrence, and poor outcomes.4
When Rb-E2F pathway alterations are present2,5:
- The normal cell cycle process is compromised
- This contributes to aggressive growth of cancer cells
- The immune system’s ability to effectively attack cancer cells is compromised, contributing to tumor progression
Meaningful advancement requires distinctly different thinking
Oncolytic immunotherapy (OIT) is a highly advanced area of research in NMIBC4,7,8:
- 3 decades of extensive research and clinical trials have established OIT as a promising treatment modality for cancer
- OIT can be engineered to address the Rb-E2F pathway alterations that are highly correlated with NMIBC
Oncolytic immunotherapy represents a highly promising approach for NMIBC
OIT leverages genetically modified viruses that conditionally replicate within cancer cells, causing cell lysis and release of viral- and tumor-specific antigens, enhancing the clinical potential through8,9 :
Selectivity: targets and kills cancer cells while sparing normal ones, minimizing off-target effects and toxicities
Amplification: efficiently spreads to and kills bystander cancer cells, amplifying the anti-tumor effect in the tumor environment
Disruption: each administration of oncolytic immunotherapy further disrupts the tumor microenvironment, making cancer cells more vulnerable to immune attack; even if cancer cells were not all eliminated during the first treatment, they might be more susceptible upon repeat administration
Stimulation: across both the innate (an immediate reaction for the viral and tumor-specific antigens) and adaptive (targeted, long-term defense) immune responses. This creates a robust, multifaceted, and durable attack against cancer
References: 1. Wang J-P, Jiao Y, Wange C-Y, et al. Rb knockdown accelerates bladder cancer progression through E2F3 activation. Int J Oncol. 2017;50:149-160. 2. Huang M-F, Wang Y-X, Chou Y-T, et al. Therapeutic strategies for RB1-deficient cancers: intersecting gene regulation and targeted therapy. Cancers. 2024:16:1558. 3. Venkadakrishnan VB, Yamada Y, Weng K, et al. Significance of RB loss in unlocking phenotypic plasticity in advanced cancers. Mol Cancer Res. 2023;21:497-510. 4. Ramesh N, Ge Y, Ennist DL, et al. CG0070, a conditionally replicating granulocyte macrophage colony-stimulating factor—armed oncolytic adenovirus for the treatment of bladder cancer. Clin Cancer Res. 2006;12(1):305-313. 5. Kitajima S, Li F, Takahashi C. Tumor milieu controlled by RB tumor suppressor. Int J Mol Sci. 2020;21:2450. 6. Laine A, Westermarck J. Molecular pathways: harnessing E2F1 regulation for prosenescence therapy in p53-defective cancer cells. Clin Cancer Res. 2014;20(14):3644-3650. 7. Rahman MM, McFadden G. Oncolytic viruses: newest frontier for cancer immunotherapy. Cancers. 2021;13:5452. 8. Yan Z, Zhang Z, Chen Y, et al. Enhancing cancer therapy: the integration of oncolytic virus therapy with diverse treatments. Cancer Cell Int. 2024;24:242. 9. Zhang Y, Li Y, Chen K, et al. Oncolytic virotherapy reverses the immunosuppressive tumor microenvironment and its potential in combination with immunotherapy. Cancer Cell Int. 2021;21:262.