The treatment landscape needs more bladder-sparing agents that work for more patients
There are approximately
84,870 new
bladder cancer
cases annually1
3 out of 4 are NMIBC,
making it the most common form
of bladder cancer2
Patients with newly
diagnosed high-risk
NMIBC have a4:
- 60%-70% chance of recurrence
- 10%-45% chance of progression to muscle-invasive or metastatic bladder cancer within 5 years
40% are high-risk
disease3
- A diagnosis characterized by high rates of recurrence and progression4
BCG fails ~50% of patients with NMIBC5
Facets of BCG failure
The FDA defines BCG-unresponsive disease (BCG failure) as one of the following7:
- Persistent or recurrent CIS ± Ta/T1 disease within 12 months of adequate BCG therapy
- Recurrent high-grade Ta/T1* disease within 6 months of adequate BCG therapy
- T1 high-grade disease at the first evaluation following an induction BCG course
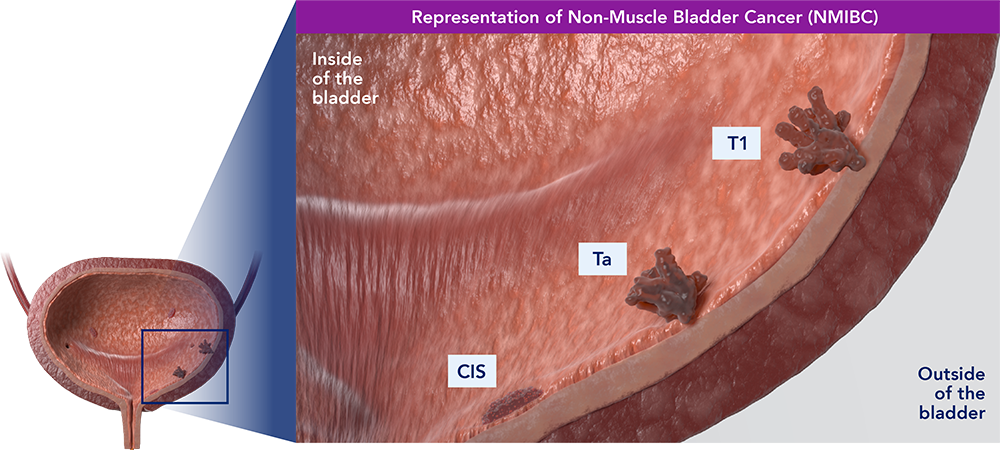
Ta refers to noninvasive papillary carcinoma. This type of growth is often found on a small section of tissue that can typically be easily removed with TURBT. A tumor stage of T1 refers to a tumor with superficial invasion of the lamina propria that has not penetrated the muscular wall of the bladder.
Radical cystectomy is currently considered the gold standard in the high-risk, BCG-unresponsive NMIBC setting
Risks
Radical cystectomy is associated with a high complication rate, morbidity, mortality, and substantial long-term costs8,9
Physical burden
Many patients are unable or unwilling to undergo this demanding, and potentially life-altering procedure6:
- Patients tend to be older6
- May have residual pain and side effects from repeated transurethral resection of bladder tumors (TURBT) and intravesical therapy10-12
- May not be physically fit enough for radical cystectomy6
Psychological burden
Patients with bladder cancer experience high rates of anxiety, depression, and mental illness13,14:
- Poor prognosis and/or treatment outcomes can exacerbate mental health issues, as can radical cystectomy14,15
- Those who undergo radical cystectomy often suffer from reduced body image, pain, and sexual dysfunction15
- For women who undergo radical cystectomy, removal of reproductive organs, when necessary, can carry additional risks and trauma16,17
A significant number of patients are unwilling or unable to undergo radical cystectomy18
The BCG shortage further limits options for achieving favorable outcomes
An estimated 8,000 patients/year do not receive optimal care due to the BCG shortage19
Access and availability can make it difficult, or in some cases impossible, to adhere to guideline recommendations20
- These real-world challenges have also been observed in the clinical trial setting, where a substantial proportion of patients are not receiving adequate BCG as defined by the FDA7,20,21
Supply issues may result in patients receiving intravesical chemotherapy, or a lower dose of intravesical BCG, which has been associated with a greater risk of recurrence and progression21,22
In the absence of access to intravesical BCG, patients may be encouraged to undergo early radical cystectomy to mitigate the risk of recurrence and progression20
Impact of the BCG shortage
Ashish M. Kamat, M.D. MBBS. Endowed Professor of Urologic Oncology (Surgery) and Cancer Research at University of Texas MD Anderson Cancer Center discusses the history and impact of the BCG shortage in the US and life-altering impact on patients.
Transcript: The BCG shortage has been going on for quite some time, and over the years we have had the fortune, or I guess the misfortune in many ways to find the impact of the BCG shortage on patients. That in areas that had ongoing BCG shortages, the incidents of radical cystectomies went up almost 300%, which means that patients who did not have access to BCG to treat their high-risk non-muscle invasive bladder cancer were undergoing radical cystectomy, which is a life altering surgery, 300 times more often than regions that had BCG available. And of course, that’s literally what’s happening in the United States. There are regions where patients don’t have access to BCG. They have to try other therapies which are not as good as BCG. And because of that, we’re seeing an increase in recurrences of these high-risk tumors in these patients.
It’s time to radically change how precious bladders are treated
Take a closer look at a key mechanism involved in bladder cancer
To learn more about the CG Oncology clinical program,
contact Medical Affairs at medicalaffairs@cgoncology.com
BCG=bacillus Calmette-Guérin; CIS=carcinoma in situ; FDA=Food and Drug Administration; IBCG=International Bladder Cancer Group; NMIBC=non-muscle invasive bladder cancer; TURBT=Transurethral resection of bladder tumor.
The information on this site is intended for audiences in the United States only. The content on this site may not apply to non-U.S. audiences as regulatory control, legal requirements, and/or medical practices may vary in other countries.
The content on this website is for your information. It is not intended to be a substitute for professional medical advice, diagnosis or treatment.
REFERENCES:
1. National Cancer Institute SEER Program. Cancer Facts and Figures 2024. Accessed November 27, 2024. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2024/2024-cancer-facts-and-figures-acs.pdf
2. Holzbeierlein JM, Bixler BR, Buckley DI, et al. Diagnosis and treatment of non-muscle invasive bladder cancer: AUA/SUO Guideline: 2024 Amendment. J Urology. 2024;211(4):533-538.
3. Data on file. CG Oncology, Inc.
4. Sylvester RJ, van der Meijden APM, Oosterlinck W, et al. Predicting recurrence and progression in individual patients with stage TA T1 bladder cancer using EORTC risk tables: a combined analysis of 2596 patients from seven EORTC trials. European Urology. 2006;49(3):466-477.
5. Rouanne M, Adam J, Radulescu C, et al. BCG therapy downregulates HLA-I on malignant cells to subvert antitumor immune responses in bladder cancer. J Clin Invest. 2022;132(12):e145666.
6. Lebacle C, Loriot Y, Irani J. BCG unresponsive high-grade non muscle invasive bladder cancer: what does the practicing urologist need to know? World J Urol. 2021;39(11):4037-4046.
7. BCG-unresponsive nonmuscle invasive bladder cancer: developing drug and biological products for treatment: guidance for industry. 2024. Accessed November 27, 2024. https://www.fda.gov/media/101468/download
8. Maibom SL, Joensen UN, Poulsen AM, Kehlet H, Brasso K, Røder MA. Short-term morbidity and mortality following radical cystectomy: a systematic review. BMJopen. 2021;11(4):p.e043266.
9. Berger I, Xia L, Wirtalla C, Dowzicky P, Guzzo TJ, Kelz RR. 30-day readmission after radical cystectomy: identifying targets for improvement using the phases of surgical care. Can Urol Assoc J. 2019;13(7):E190-E201.
10. Understanding TURBT: Bladder Tumor Removal Surgery. BCAN—Bladder Cancer Advocacy Network. Updated June 6, 2022. Accessed November 27, 2024. bcan.org/bladder-cancer-turbt
11. Kim LHC and Patel M. Transurethral resection of bladder tumour (TURBT). Transl Androl Urol. 2020;9(6):3056-3072.
12. Li Y, Yousseh SF, Buanz ABM. Intravesical combination therapies for non-muscle invasive bladder cancer: recent advances and future directions. Eu J Pharmacol. 2022;926:175024.
13. Vartolomei L, Vartolomei MD, Shariat SF. Bladder cancer: depression, anxiety, and suicidality among the highest risk oncology patients. Eur Urol Focus. 2020;6(6):1158-1161.
14. Pham H, Torres H, Sharma P. Mental health implications in bladder cancer patients: a review. Urol Oncol. 2019;37(2):97-107.
15. Rogers Z, Glaser A, Catto JWF, et al. Health related quality of life after a diagnosis of bladder cancer: a longitudinal survey over the first year. BJU Int. 2024;133(4):460-473.
16. Richter LA, Egan J, Alagha EC, Handa VL. Vaginal complications after radical cystectomy for bladder cancer: a systematic review. Urology. 2021;156:e20-e29.
17. Choi H, Park JY, Bae JH, Tae BS. Health related quality of life after radical cystectomy. Transl Androl Urol. 2020;9(6):2997-3006.
18. Nazmifer M, Williams C, Naser Tavakolian A, et al. Clinical and preclinical therapies for bladder cancer following bacillus Calmette-Guérin failure. J Urology. 2022;209(1):32-48.
19. Chiujdea S, Ferro M, Vartolomei MD, et al. Epirubicin and nonmuscle invasive bladder cancer treatment: a systematic review. J Clin Med. 2024;13(13):3789.
20. Mori K, Miura N, Babjuk M, et al. Low compliance to guidelines in nonmuscle invasive bladder carcinoma: a systematic review. Urol Oncol. 2020;38(10):774-782.
21. Ostrowski DA, Chelluri RR, Herzig M, et al. Diminished short-term efficacy of reduced-dose induction BCG in the treatment of non-muscle invasive bladder cancer. Cancers (Basel). 2023;15(14):3746.
22. Ourfali S, Ohannessian R, Fassi Fehri H, Pages A, Badet L, Colombel M. Recurrence rate and cost consequence of the shortage of Bacillus Calmette-Guérin Connaught strain for bladder cancer patients. Eur Urol Focus. 2021;7(1):111-116.
