A significant unmet need in HR NMIBC: achieving durable efficacy and tolerability with a bladder-sparing agent
Bacillus Calmette-Guérin (BCG) fails ~50% of patients with high-risk NMIBC3
While many patients may initially respond to an induction course of this mainstay therapy, approximately half will experience disease recurrence.4
The FDA defines BCG-unresponsive disease (BCG failure) as one of the following6:
Persistent or recurrent
disease within 12 months of adequate BCG therapy
Recurrent high-grade
disease within 6 months of adequate BCG therapy
disease at the first evaluation following an induction BCG course
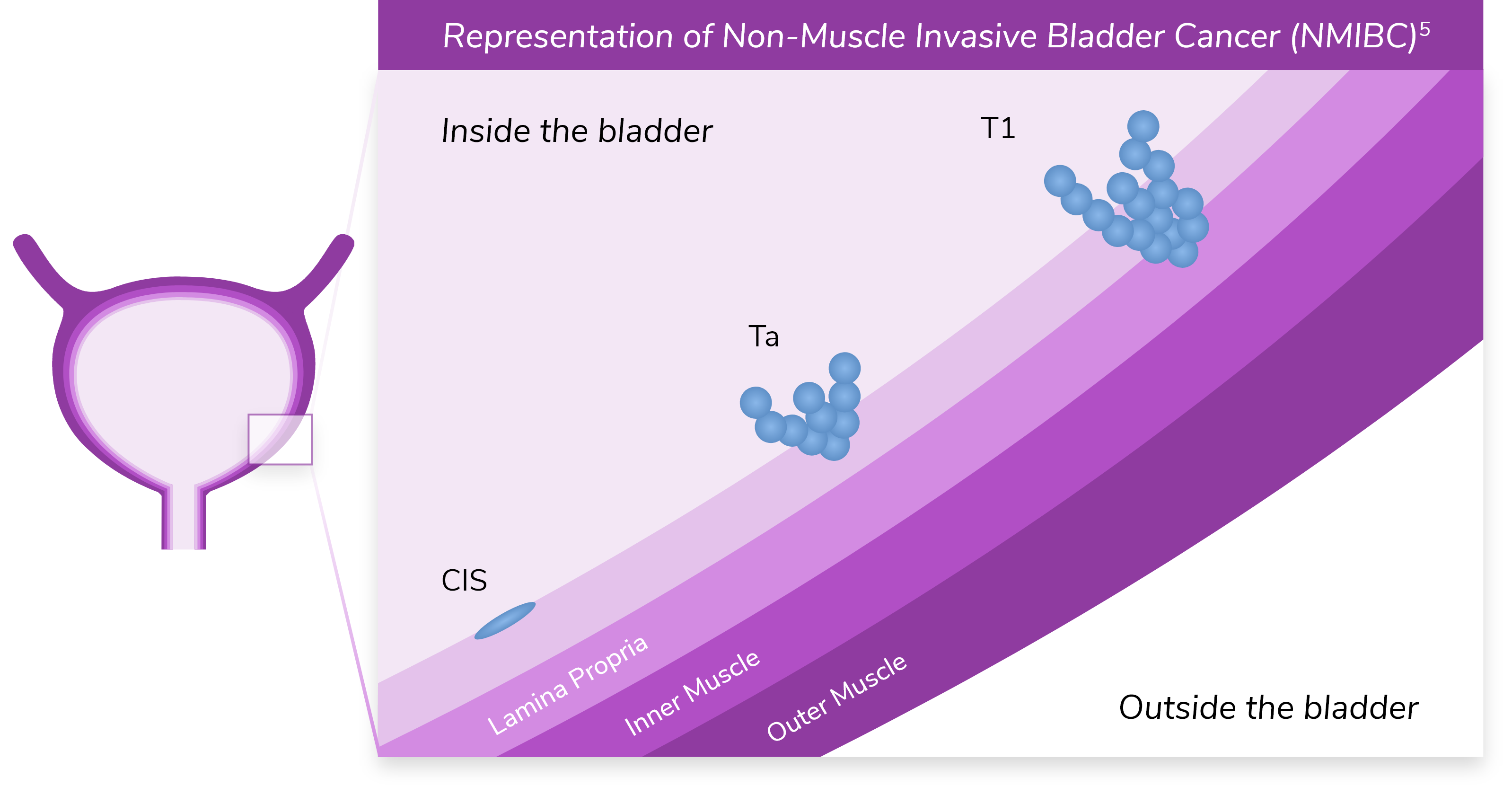
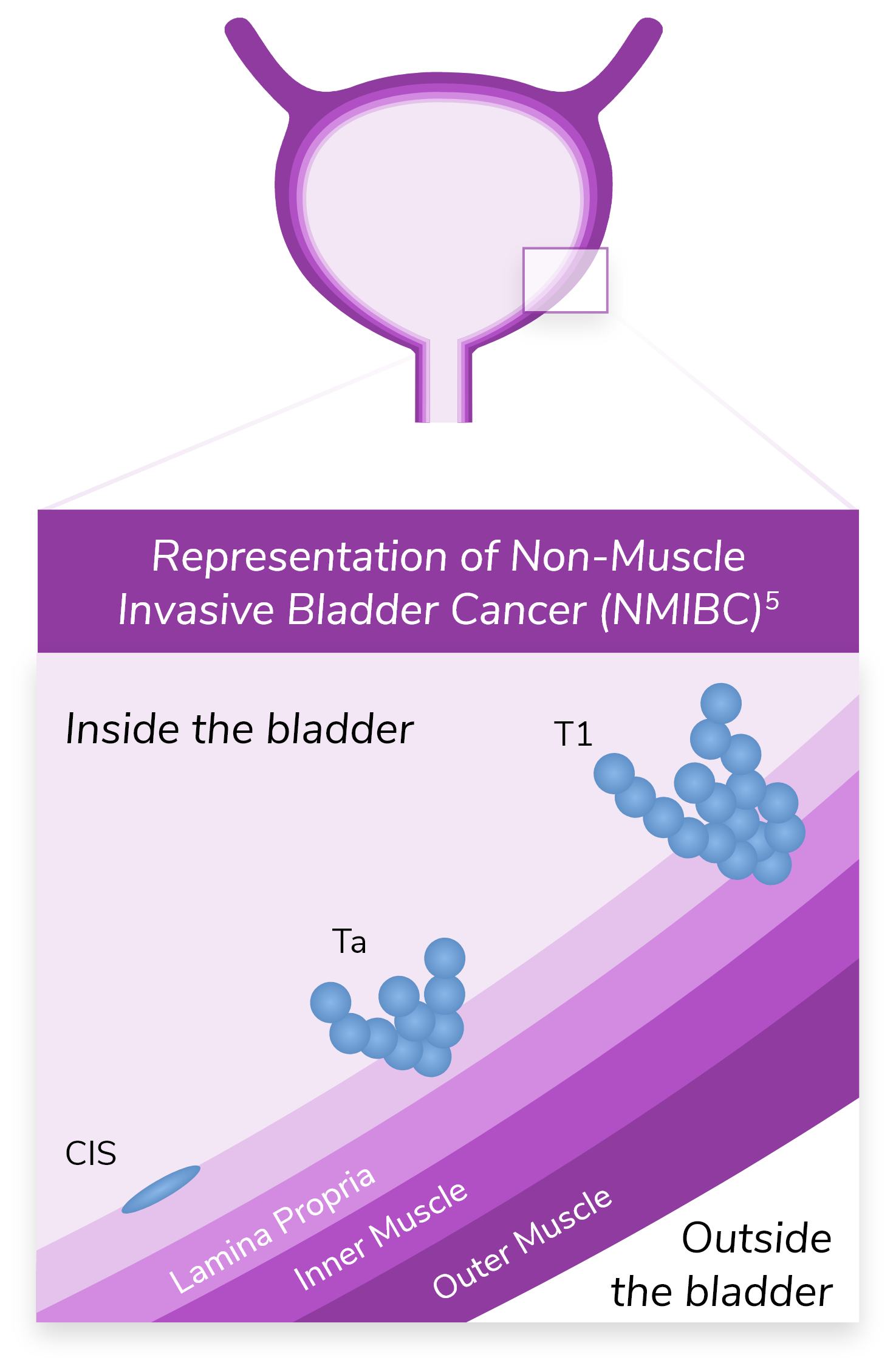
*Ta refers to noninvasive papillary carcinoma. T1 refers to a tumor that has invaded the lamina propria but has not penetrated the muscular wall of the bladder.5
Radical cystectomy (RC) carries a heavy burden
While RC is considered the gold standard for managing HR BCG-UR NMIBC, it is associated with7-9:
- A high complication rate
- Morbidity
- Mortality
- High long-term costs
- Reduced body image and sexual function
Bladder-sparing treatment options fall into various categories2:
Intravesical
chemotherapy
Systemic
immunotherapy
Gene therapy
IL-15
receptor agonist
Treatments in
development
Shaping the future of bladder cancer treatment
With all the existing burdens of disease surveillance, and the burdens of current therapy on patients, it’s essential that the next step in a patient with bladder cancer’s life be one that considers the challenges they have faced—and will continue to face.
A refined therapy for NMIBC:
- Is dual-acting and selectively targets bladder cancer cells10,11
- Does not require combination with BCG12,13
- Improves efficacy and durability of response13,17
- Leverages familiar intravesical administration procedures and schedules that easily integrate into current urology practice18,19
- Has a safety and tolerability profile that helps mitigate the barriers to improved potential outcomes13-16


